Der vorliegende Beitrag entstand im Rahmen der Sommerakademie der Schweizerischen Studienstiftung und wurde redaktionell begleitet von Reatch.
We've all heard about bacteria—tiny organisms some of which can make us sick—and how they're getting better at surviving our drugs. These superbugs, also known as antibiotic-resistant bacteria (AMR), are becoming a major threat to public health. The situation is serious, as it is estimated that AMR infections resulted in 4.95 million deaths in 2019 alone, with some experts predicting that by 2050, drug-resistant infections could cause 10 million deaths annually1,2. If true, this would mark the most significant health crisis of our time. While such predictions should be considered with caution, they highlight just how serious the problem is. So, are we out of options?
Let me introduce you to a fascinating, even smaller organism: the bacteriophage. Its name means "bacteria eater3" and that’s exactly what these tiny things do—they infect and destroy bacteria. As bacteria's natural predators, bacteriophages offer a glimmer of hope in the fight against antibiotic-resistant infections. Imagine you have a burn wound on your arm. Unfortunately, it becomes infected with a bacterial strain resistant to all available antibiotics. Your doctor might inform you that while antibiotics are no longer effective, there's another option: phage therapy. The idea is simple but powerful: applying a solution or patch containing bacteriophages to the wound would allow these little organisms to target and eliminate the antibiotic-resistant bacteria, ultimately giving your body the chance to heal.
Phage therapy has several advantages over traditional antibiotics. For one, phages are very specific—they only target the harmful bacteria without damaging your own cells or the beneficial bacteria in your body (your microbiota) 4–6 , like the ones in your gut helping you with digestion. Classical antibiotics, on the other hand, not only affect the bad bacteria but also the general microbiome4, which can lead to side effects. This precision makes phage therapy a highly favorable treatment. However, this specificity also presents a challenge: since each phage targets one specific bacterial strain, it can't be used as a one-size-fits-all treatment. Each patient's infection is slightly different, with a combination of different sub strains of a bacterium. This means we can’t treat multiple patients with the same phages, but rather need to personalize it7,8. Hence for now, phage therapy isn’t an off-the-shelf solution—but researchers are working hard to make that a reality.
There are ways to develop phage therapy that tackles a broad range of bacterial strains within a single species and develop it to an off-the-shelf drug. Options include making cocktails of different phages or engineering them. In a phage cocktail, multiple phages with different specificities are combined into a single treatment, allowing the cocktail to target and eliminate a broader spectrum of bacteria compared to a single phage. Alternatively, phage engineering involves modifying a single phage to infect a wider variety of bacterial strains. One approach is to alter the phage's tail fibers—the "feet" that recognize and attach to bacteria. By engineering these fibers, the phage can bind to different bacterial targets, inject its genetic material, and trigger bacterial lysis, leading to the destruction of the host. Both strategies—cocktails and engineered phages—are areas of ongoing research as scientists continue to refine and optimize these treatments.
Just as bacteria can develop resistance to antibiotics, they unfortunately can also become resistant to phages. To evade phage infection, bacteria have evolved several defense mechanisms. One such strategy involves altering their surface so that the feet of the phage can no longer recognize and attach to them. Additionally, bacteria have developed sophisticated immune systems to defend against phages. A well-known example is the CRISPR-Cas system, which is now widely used as a gene-editing tool but originally evolved in nature as a bacterial defense mechanism. It acts like molecular scissors, identifying and cutting the genetic material of invading phages to prevent infection. In response, phages are constantly evolving to counteract bacterial defenses. They may modify their feet or develop mechanisms to evade the bacterial immune system. In the field of phage therapy, researchers aim to engineer phages to maximize their effectiveness and reduce the development of resistance. This can involve improving the phage feet for better recognition or incorporating genes that help phages bypass bacterial defenses. Additionally, using a cocktail of different phages is also an effective strategy to combat resistance, as it requires bacteria to simultaneously develop resistance to multiple phage types, a much more challenging task.
The good news is phage therapy is already saving lives through personalized medicine approaches. Let’s hear the story of Joel Grimwood9. He needed a heart transplant, but the ventricular assist device in his heart was infected with a drug-resistant bacterial colony. Multiple transplant centers turned him down because the infection couldn’t be cleared, making the transplant impossible. Fortunately, when Joel was finally treated with a personalized, experimental phage therapy by UC San Diego Health, the infection cleared, allowing him to receive a new heart—ultimately saving his life. While phage therapy isn’t yet available as a standard treatment for everyone, it already saves many lives every year in cases where antibiotics fail, proving its effectiveness.
Interestingly, the idea of phage therapy is remarkably old. Phages were discovered in the 1920s during the pre-antibiotic era and were among the first tools used to combat bacterial infections. Early, somewhat primitive applications included serum treatments for diseases like pneumococcal infections and diphtheria. Notably, Sinclair Lewis even referenced the use of phages to treat bubonic plague on a Caribbean island10. However, with the discovery of penicillin as the first antibiotic, interest in phage research largely declined in most parts of the world. Nevertheless, some former USSR countries, particularly Poland, Georgia, and Russia, preserved and advanced their expertise in phage therapy over the decades. For instance, the Elica Phage Therapy Center (EPTC) in Georgia treated 400 patients with phages in 201811. While phage therapy is an old idea, it is now being intensely investigated again because of the growing antibiotic crisis, with improvements in the therapy aiming to move from a personalized approach to a larger-scale application.
To conclude, while the rise of antibiotic-resistant bacteria is undeniably a serious threat, we are far from out of options. Bacteriophages, with their natural ability to infect and destroy harmful bacteria, offer an exciting and innovative approach to treating infections that no longer respond to traditional antibiotics. Phage therapy, although not yet a mainstream treatment, is already saving lives and holds enormous potential for the future of medicine. As research continues and phage therapy advances, bacteriophages may soon become a powerful tool in our battle against drug-resistant infections. The future is full of promise, and the battle against superbugs is far from over.
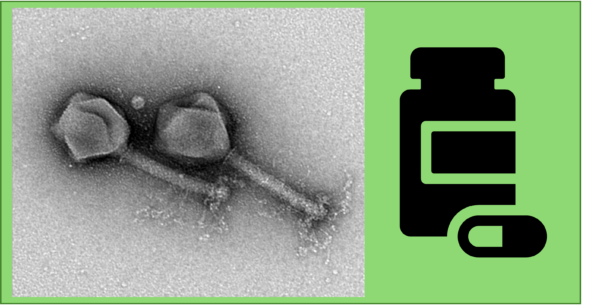
Title picture: TEM image courtesy of M. Hajfathalian.
1. Murray CJL, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022;399(10325):629-655. doi:10.1016/S0140-6736(21)02724-0
2. O’Neill J. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. Published online December 2014. https://amr-review.org/sites/d...
3. Wright A, Hawkins CH, Änggård EE, Harper DR. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic‐resistant Pseudomonas aeruginosa ; a preliminary report of efficacy. Clinical Otolaryngology. 2009;34(4):349-357. doi:10.1111/j.1749-4486.2009.01973.x
4. Hibstu Z, Belew H, Akelew Y, Mengist HM. Phage Therapy: A Different Approach to Fight Bacterial Infections. Biologics. 2022;16:173-186. doi:10.2147/BTT.S381237
5. Alqahtani A. Bacteriophage treatment as an alternative therapy for multidrug-resistant bacteria. SMJ. 2023;44(12):1222-1231. doi:10.15537/smj.2023.44.12.20230366
6. Müller DM, Pourtois JD, Kim MK, et al. Bacterial Receptors but Not Anti-Phage Defence Mechanisms Determine Host Range for a Pair of Pseudomonas aeruginosa Lytic Phages. Published online May 1, 2024. doi:10.1101/2024.04.30.591980
7. Abedon ST, Danis-Wlodarczyk KM, Wozniak DJ. Phage Cocktail Development for Bacteriophage Therapy: Toward Improving Spectrum of Activity Breadth and Depth. Pharmaceuticals (Basel). 2021;14(10):1019. doi:10.3390/ph14101019
8. Kim MK, Chen Q, Echterhof A, et al. A Blueprint for Broadly Effective Bacteriophage Therapy Against Bacterial Infections. Published online April 21, 2024. doi:10.1101/2024.04.20.590411
9. Turning a Phage: Innovative Therapy Clears Infection and Allows Heart Transplantion. Center for Innovative Phage Applications & Therapeutics (IPATH) Accessed September 30, 2024. https://youtu.be/785pviCyFq0
10. Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014;5(1):226-235. doi:10.4161/viru.25991
11. Sacher J. Evergreen Phage 2019: Meeting Recap. https://phage.directory/capsid/evergreen-2019-recap
Die Beiträge auf dem Reatch-Blog geben die persönliche Meinung der Autor*innen wieder und entsprechen nicht zwingend derjenigen von Reatch oder seiner Mitglieder.

Comments (0)